|
Ruxolitinib
Peri-transplant Administration of Ruxolitinib in MF
In May 2019, the label for the JAK1/2 inhibitor, ruxolitinib, was expanded for use in steroid-refractory acute graft-versus-host disease (GvHD).1 Findings from a pilot open-label study showed positive results when administered peri-transplant in combination with reduced intensity conditioning (RIC).2 The trial was designed to evaluate ruxolitinib at two doses, 5 mg and 10 mg, which was given at day -3 pre-transplant and continuously through day +30, and then tapered off by day +33. The primary endpoint was safety, and the objective was to identify the maximum tolerated dose (MTD) and phase 2 dose for investigation.
Six patients were enrolled on each treatment arm.2 For all patients, the 1-year overall survival (OS) was 80%, and the median progression-free survival (PFS) was 68%; non-relapse mortality was 21%. Importantly, all patients were grafted, with a median time to engraftment of 16 to 19 days. There were no hematologic dose-limiting toxicities. Grade ≥3 adverse events were observed, which did not appear to be related to ruxolitinib, including cardiac, pulmonary, and gastrointestinal in the 5 mg group. There was one case in the 10 mg group of grade 3 or higher lung toxicity. There were two deaths, one from respiratory failure in the 5 mg group and one from acute GvHD in the 10 mg group. The median time to onset of acute GvHD was 20 days in the 5 mg group and 51 days in the 10 mg group. Grade 3 to 4 acute GvHD was uncommon, seen in only one patient overall.
What is the clinical relevance of these data?
Dr. Prithviraj Bose: “In general, what we do now is that we taper off the ruxolitinib (rux) over 5-7 days, and basically give the last dose the day before they start conditioning. And now here, we have an example of giving it right through the transplant. Ruben, your thoughts?”
Dr. Ruben Mesa: “Well, I think that’s an important study really for a variety of reasons. First, the stoppage of rux pre-transplant always introduces another variable; because there can be disease rebound and increase in cytokines, patients feel worse, and there were examples of patients having some amount of a withdrawal syndrome that can occur as well. So, there's a variety of negatives that was stopping ruxolitinib and indeed, we've learned in many ways, although there's conditioning that starts on day one, the amount of fibrosis is not gone. There are a variety of reasons to think that continuation of rux even from the myelofibrosis end probably makes sense to do throughout the transplant process. This I think helps further validate some of the safety around that and it may have some benefits as it relates to GvHD. We know the process of a patient getting cured of myelofibrosis is not an instant one. With JAK2, a little burden tends to go away, but it usually takes several months. It takes months for the fibrosis to resolve, for the spleen to shrink. I think the evolution of ruxolitinib continuing on through the peritransplant process, and eventually being tapered months after the transplant, probably is where we will likely end up, that may be more beneficial for the patients, it may be more biologically accurate than just acutely stopping the drug.”
Alternative Ruxolitinib Dosing in MF Patients with Anemia: The REALISE Trial
The REALISE trial was developed to test ruxolitinib at a starting dose of 10 mg twice weekly for 12 weeks with gradual up-titration to 15 mg to 20 mg in patients with MF and anemia.3 Findings from this phase 2, open-label trial, single-arm study showed that this alternative dosing regimen produced comparable efficacy and safety results to those of the original ruxolitinib trials4,5 and may be an option for patients who develop anemia early after starting ruxolitinib therapy.
The primary endpoint was the proportion of patients achieving a reduction in spleen length (SL) of at least 50% at week 24.3 The trial included 51 patients with a median duration of ruxolitinib exposure of 38 weeks. The median dose was 20 mg/day. At week 24, 28 of the 44 evaluable patients had reduction in SL of ≥50%, and five patients had an SL reduction of 25%-50%. Of the eight patients who were transfusion dependent at baseline, six (75%) had an SL reduction of ≥50% at week 24. For the 25 patients who were non-transfusion dependent at baseline, SL reductions were observed in 17 (70.8%) patients. Hemoglobin levels dropped in the first 8 weeks of treatment, then stabilized, and platelet levels remained constant. At data cutoff, 32 patients were still undergoing treatment, and 19 had discontinued. Most dose reductions or interruptions were due to adverse events, which were mainly hematological (eg, anemia and thrombocytopenia). Improvements in symptoms were also seen in patient-reported outcomes at week 24.
What do these findings mean to current practice?
Dr. Prithviraj Bose: “I think it's very reassuring that doing it this way preserves the spleen response rate. Granted, this was by palpation, but it looks pretty solid. And we've always felt that we need to optimize the dose of rux to get the best spleen response, but this tells us that if we do it for 12 weeks at a moderate dose and then go up, we are still where we want to be. I think that's very useful and very, very, very reassuring for those who already do that.”
Dr. Ruben Mesa: “I think that dose intensity is important. Dose intensity over time is important. It may not need to be present at day one. but trying to get patients to that 15 mg or 20 mg twice-a-day dose really does matter. One thing I've observed in the patients referred to me is that there's not an insignificant number of patients that really are underdosed with ruxolitinib.”
Fedratinib
Re-analysis of the JAKARTA-2 Trial
Fedratinib was approved in August 2019 for patients with intermediate-2 or high-risk primary or secondary (post-polycythemia vera [PV] or post-essential thrombocythemia [EV]) MF. This agent selectively inhibits JAK2/FLT as well as RET and was approved based on findings from the JAKARTA-1 and -2 trials.6,7 Notably, these studies included patients with platelet counts of ≥50 x 109/L, which is lower than the inclusion criterion of ≥100 x 109/L in the ruxolitinib COMFORT trials.4,5
A re-analysis of JAKARTA-2 was conducted to apply the more stringent definitions of ruxolitinib resistance and intolerance to the overall study population.8 In this analysis, 79 of the 97 enrolled patients met the new criteria for ruxolitinib resistance (n=62; relapsed: n=18; refractory: n=47) and intolerance (n=14). Recently presented results showed that rates of spleen volume response (SVR) were 28%, 31%, and 29% in patients who had disease that was relapsed, refractory, and intolerant to ruxolitinib, respectively. Toxicities were consistent with prior reports, with no new safety signals identified. In addition, recent findings indicate that fedratinib elicited robust spleen response rates, regardless of the reason for ruxolitinib discontinuation.9,10 An analysis of health-related quality of life (HRQoL) benefit showed that fedratinib provided clinically meaningful improvement across patient subgroups.11,12
What is the role of fedratinib in the current treatment algorithm for MF?
Dr. Ruben Mesa: “So in aggregate, we see that even with a fresh look at the data from the fedratinib trials, we see that first, in the frontline setting, this agent is clearly efficacious. Data clearly show that the drug can be used at the full dose of 400 mg daily in patients with platelet counts of 50,000 to 100,000/L, and that it significantly improves splenomegaly and symptoms vs placebo. Second, we see that in the second-line setting, again, either with thrombocytopenia or whether or not there's efficacy, there's safety and even in a more stringent cohort of patients, the efficacy is at least a third for patients who have been previously treated with ruxolitinib. There are several different clinical aspects related to this. But my key takeaways for colleagues as they asked me about this is one without question, we immediately have data which shows that fedratinib is an important second-line consideration. Without question, there are patients out there who are being treated now with ruxolitinib and that due to the lack of another option, or unwillingness or being ineligible for clinical trials, remain on ruxolitinib. So right away, it's an option for them and certainly the updated NCCN Guidelines - and I believe you, Dr. Bose, are one of the contributors to those guidelines - clearly views it in that setting. Second, in the frontline setting certainly has that approval. Surely these data raise potentially some differentiation by being able to be used in full dose in individuals with a platelet count between 50 and 100,000.
Now a couple of practical points to raise for the community oncologist regarding its use, it's all overcomeable. Fedratinib is straightforward to use, but there are a couple additional considerations. First, there is a black box warning based on a very low rate, less than 1%, of neurologic events, like the Wernicke’s that occurred in the earlier studies.”
Dr. Prithviraj Bose: “I think it provides an important new option, as you said, in patients who failed ruxolitinib. I'm glad that we have at least a framework of defining ruxolitinib failure provided by these more stringent criteria that were used in the reanalysis. And it is very reassuring to see that even with those rigorous criteria, you have this 30% rate of SVR and a 27% rate of total symptom score (TSS) improvement, that makes it a pretty solid drug in the second-line setting regarding possible frontline use. Again, we have years of data with rux. We are comfortable with it. It has a survival benefit. So, I think the rux remains the cornerstone of frontline therapy. But the point you made about the platelet counts in the 50 to 100 range where the rux label would suggest using only 5 BID. There, the fact that the efficacy here is the same and the dose is the same. That's very intriguing, and now again, you and I probably are using 10 BID in those patients, but at the same time, we are probably not using the most efficacious dose of rux. Whereas here, it seems like we can do that. I think that's very interesting.”
Recommendations in the management of patients on fedratinib therapy:
- Check thiamine levels before stating therapy and advise patients to take thiamine supplements while on therapy
- Prescribe antiemetic or anti-diarrheal medications when needed to treat GI side effects
Enrolling fedratinib clinical trial of note:
NCT03952039: An Efficacy and Safety Study of Fedratinib Compared to Best Available Therapy in Subjects With DIPSS-intermediate or High-risk Primary Myelofibrosis, Post-polycythemia Vera Myelofibrosis, or Post-essential Thrombocythemia Myelofibrosis and Previously Treated With Ruxolitinib (FREEDOM2)
Pacritinib
Pacritinib is an oral JAK2/FLT3/IRAK inhibitor that is in late-stage development in both the front- (PERSIST-1) and second-line (PERSIST-2) treatment of MF. Unlike ruxolitinib and fedratinib, pacritinib may be used at full dose in patients with marked thrombocytopenia. A pooled analysis of these studies was recently conducted to assess the optimal dose of pacritinib and confirm the safety and efficacy of this agent.13
PERSIST-1 was conducted among patients with intermediate-2 and high-risk MF; there was no platelet threshold or cut off to enroll on the study.14 Pacritinib was given at a dose of 400 mg once daily, and results showed an overall spleen response of 25%. Among those with platelets <100 x 109/L, spleen response was 24% and in patients with platelets <50 x 109/L, spleen responses were observed in 33%. Overall, the TSS reduction was 19%. Anemia and thrombocytopenia were observed in 17% and 21%, respectively, of patients, similar to what was observed with fedratinib.
The phase 3 PERSIST-2 trial evaluated pacritinib at two doses: 400 mg once daily and 200 mg twice daily; versus BAT, including ruxolitinib.15 Patients who received prior treatment with one or two JAK inhibitors were allowed to enroll. Results showed that more patients receiving pacritinib 200 mg had higher rates of spleen response and reduction in TSS versus those on the 400 mg dose or on best available therapy (BAT).
As shown in Table 1, findings from the pooled analysis showed that pacritinib at the 200 mg dose was more efficacious versus the 400 mg dose in terms of SVR and TSS.
Table 1. Results of the Pooled Analysis of the PERSIST-1 and PERSIST-2 Studies.13
Measure |
Pooled PAC 400 mg QD and 200 mg BID |
Pooled PAC |
PAC |
Pooled BAT (Including Ruxolitinib) |
SVR |
||||
N, ITT efficacy population |
104 |
73 |
31 |
48 |
N (%) with ≥35% SVR |
24 (23%) |
15 (21%) |
9 (29%) |
1 (2%) |
Difference PAC-BAT, % (CI) |
21.0 (9.8, 29.3) |
18.5 (5.0, 29.6) |
25.9 (4.3, 44.5) |
-- |
P value vs BAT |
.0007 |
.0025 |
.0059a |
-- |
TSS |
|
|
|
|
N, ITT efficacy population |
80 |
49 |
31 |
37 |
N (%) with ≥50% improvement in TSS |
16 (20%) |
9 (18%) |
7 (23%) |
4 (11%) |
Difference PAC-BAT, % (CI) |
9.2 (-5.8, 21.6) |
7.6 (-10.5, 24.1) |
10.1 (-12.0, 31.1) |
-- |
P value vs BAT |
.2944 |
.3793 |
.3372b |
-- |
aP values are compared to BAT from the PERSIST-2 study. 1/32 BAT patients with platelets <50,000/μL had a ≥35% SVR in PERSIST-2.
bP values are compared to BAT from the PERSIST-2 study. 4/32 BAT patients with platelets <50,000/μL had a >50% improvement in TSS in PERSIST-2.
BAT=best available therapy; CI=confidence interval; PAC=pacritinib; SVR=spleen volume reduction; TSS=total symptom score.
Regarding safety, gastrointestinal (GI) events were the most common treatment-emergent adverse event (TEAE); these were primarily grade 1/2 and rarely required dose reduction or interruption.13 The rates of grade 3/4 hemorrhages and deaths on study were comparable between the pacritinib and BAT treatment arms. Interestingly, the rates of grade 3/4 cardiac events were lower with pacritinib (8%) vs BAT (12%).
Dr. Ruben Mesa: “We had wondered whether by including patients with marked thrombocytopenia if we were just treating a more elite group of patients as a baseline (ie, as an initial study population for pacritinib). And this pooled analysis would tend to suggest exactly that, that again, if we're going to treat patients that have very advanced disease, there's a certain rate of them that are going to have [cardiac] events occur, but nothing that gives us a signal that there is an increased risk related to the pacritinib itself.”
PAC023: Phase 2 Dose-finding Study of the Recommended Pacritinib Dose in the Second-line Setting
PAC023 was a randomized phase 2 trial that was designed to determine the recommended dose of pacritinib in patients with intermediate- or high-risk MF.16 Patients were randomly assigned in a 1:1:1 fashion to receive pacritinib at 200 mg BID, 100 mg BID, or 100 mg QD. Patients were stratified by baseline platelet count. The primary endpoints were the proportion of patients who achieved ≥35% SVR and those with >50% TSS reduction.
A total of 161 of 164 patients were treated; results showed that pacritinib at the 200 mg BID dose elicited the most improvement in SVR (Figure 1).16
Figure 1. Percent change in spleen volume between baseline and 24 weeks by treatment arm in the PAC023 study.16
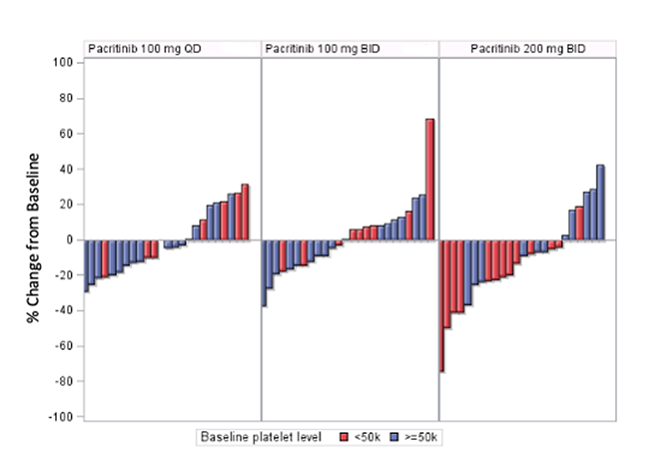
As expected, the most frequent non-hematologic TEAEs were GI and were distributed similarly among treatment arms.16 The most common hematologic TEAEs were thrombocytopenia and anemia, with the highest frequency in patients on the 200 mg BID dose, but this did not lead to higher rates of hemorrhages at the higher dose, nor were there higher rates of grade 3/4 cardiac events or infectious AEs at this dose. Based on the findings of this trial, pacritinib at the 200 mg dose was the recommended dose for this agent.
What is the clinical relevance of this trial?
Dr. Ruben Mesa: “One of my takeaways from this, as I looked at the actual response curves, was that the majority of patients at the 200 twice a day had a reduction in splenomegaly and had benefit; only 10% reached the 35% volume reduction. Now, I would tend to consider one, the 35% volume reduction was a number that we as an academic community came up with arbitrarily, it was not a validated number. And over time, we had found that individuals who had over a 10% volume reduction on ruxolitinib had a survival advantage. But I wonder if what we’ll really find over time is that, again, some much lower threshold, particularly in the second-line setting, is relevant as an endpoint in treating patients with myelofibrosis. And that if we looked at those same 200 mg twice a day, and patients with marked thrombocytopenia have a poor outcome, maybe those that have greater than a 10% volume reduction are really benefiting from the drug. And that the cut off of 35% really undersells the value of the drug.”
Dr. Prithviraj Bose: “Absolutely. Almost any degree of spleen volume reduction is meaningful clinically to a patient, certainly 10% and higher.”
Dr. Ruben Mesa: “I don't think that there's magic to the reduction of the spleen. I think that the reduction in the spleen is a biomarker of response to JAK inhibition. I think JAK inhibition has a variety of benefits to the patient. It is true that JAK inhibitors improve those things that are “superficial,” ie, splenomegaly, symptoms, etc., but I do believe that there also is likely a benefit in terms of the cytokine environment, the inflammatory markers, and the progressive kind of microenvironment niche that exists within the bone marrow. I consider those patients who demonstrate that level of response are the ones who are really responding well to JAK inhibitor therapy, and this level of response is what separates them from those who have only a 10% spleen reduction volume, which still indicates that they are benefiting from therapy. I think we'll be able to quantify that better once we have better markers of progression free survival.”
Enrolling pacritinib clinical trial of note:
NCT03165734: A Phase 2/3 Study of Pacritinib in Patients With Primary Myelofibrosis, Post Polycythemia Vera Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis (PACIFICA)
Momelotinib
Another JAK inhibitor in late-phase investigation in MF is momelotinib, a potent and selective inhibitor of JAK1/2. Like pacritinib, it also has been studied in the frontline (SIMPLIFY-1) and second-line (SIMPLIFY-2) settings. Findings from dynamic and time-to-event analyses in transfusion requirements in the SIMPLIFY-1 trial were recently presented and showed that momelotinib greatly improves transfusion burden vs ruxolitinib.17
The phase 3 SIMPLIFY-1 trial was designed to evaluate momelotinib versus ruxolitinib in ruxolitinib-naïve patients with high-risk or intermediate-2 MF.18 Patients were randomly assigned to receive either momelotinib 200 mg once daily or ruxolitinib 20 mg twice daily. Efficacy results showed that momelotinib was noninferior to ruxolitinib in regard to SVR (26.5% vs 29%, respectively; P=.11), and noninferiority was not met regarding TSS (28.4% vs 42.2%, respectively, P=.98). Results showed that momelotinib improved transfusion rate, transfusion independence, and transfusion dependence. Findings also showed an anemia benefit associated with momelotinib vs ruxolitinib (Table 2).
Table 2. Transfusion Independence and Dependence at Week 24 in the SIMPLIFY-1 Trial.18
Momelotinib |
Ruxolitinib |
P Value |
|
% TI at Week 24 |
67 |
49 |
<.001 |
% TD at Week 24 |
30 |
40 |
0019 |
% TD → TI (rolling 12-week) |
49 |
29 |
.0455 |
TD=transfusion dependence; TI=transfusion independence.
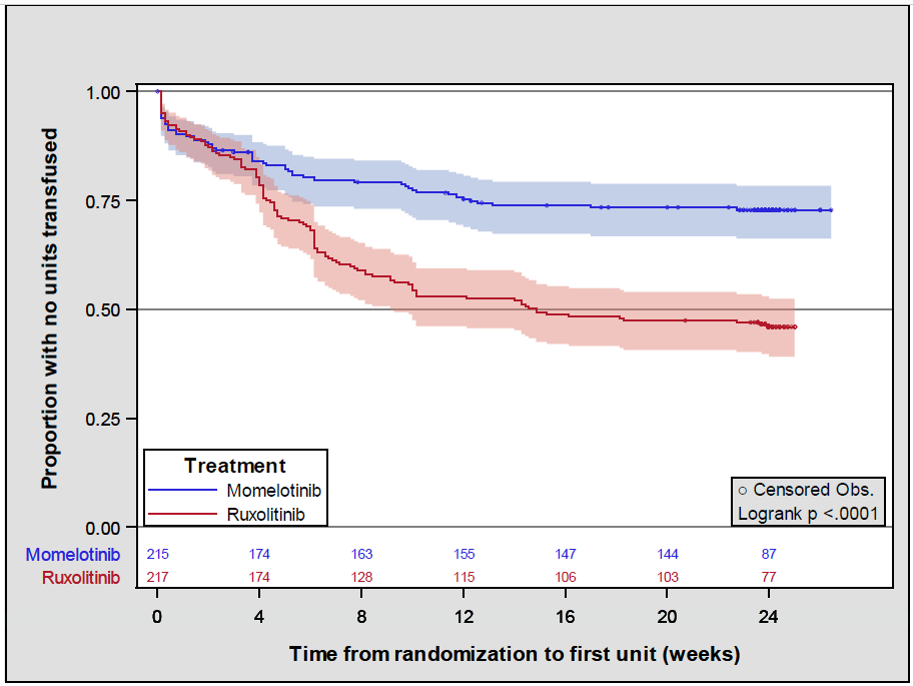
Findings from a recent analysis showed confirmed improvements in transfusion burden with momelotinib vs ruxolitinib (Figure 2).17 Moreover, the odds of receiving <3 transfusions was 3.67 times higher in the momelotinib group vs the ruxolitinib group (81% vs 54%, P=.0001), and the odds of receiving <5 transfusions was 2.99 higher for momelotinib vs ruxolitinib (83% vs 62%, respectively; P= .0001).
Figure 2. Kaplan-Meier survival function estimates for time to first red blood cell unit.17
What does the community oncologist need to know about momelotinib?
Dr. Ruben Mesa: “ I think the takeaway is there clearly is activity as it relates to momelotinib for anemia, while still having improvements in splenomegaly and symptoms. Part of this may be activity on other mechanisms, such as ACVR1 and other sites that have to do with anemia, and this may be part of the mechanism why we see momelotinib have an incremental benefit in anemia compared to fedratinib or ruxolitinib or potentially even pacritinib. So for the community oncologist, I think it's good to know that these other drugs in development, they're not quite available to them yet. But if patients have any medium, they potentially have access to centers that have this as a clinical trial. I would certainly encourage them to consider enrolling patients”
Enrolling momelotinib clinical trial of note:
NCT04173494: A Study of Momelotinib Versus Danazol in Symptomatic and Anemic Myelofibrosis Patients (MOMENTUM)
