|
Combination Approaches with Ruxolitinib
Ruxolitinib and Thalidomide in MF
The combination of ruxolitinib and thalidomide was investigated in a multicenter, two-stage, phase 2 trial that was designed to see if this approach could improve disease- and therapy-related cytopenias, as well as improve overall disease response in patients with MF.2 The trial included patients with either primary or secondary MF, and those who were receiving ruxolitinib therapy at the time of enrollment must have had a suboptimal response (eg, less than a partial response [PR] per the IWG-MRT/ELN2013 criteria) or refractory to single-agent ruxolitinib therapy. Patients must have been taking ruxolitinib for a minimum of 3 months, and on a stable dose for a minimum of 4 weeks prior to enrollment. During the run-in phase, treatment-naïve patients received single-agent ruxolitinib for 3 months, and continued to combination therapy if they achieved less than a PR. Each cycle of therapy was 28 days. The primary endpoint was the proportion of treated subjects that achieved a response by IWG-MRT criteria and by platelet response criteria.
At the time of reporting, 15 of 23 enrolled patients were evaluable.2 Results showed a significant increase in platelet count at the third cycle of the combination therapy versus baseline (P < .01), and a significant increase in platelet count was seen patients with baseline thrombocytopenia at the same timepoint (P < .05). Investigators noted a trend toward an increase in hemoglobin over successive cycles of the combination therapy. After at least 3 cycles of the combination therapy, 6 of 11 patients (56%) with available CT/MRI data had a reduction in spleen volume. The overall response rate (ORR) was 60%, and clinical improvement (ie, anemia response, symptom response, or spleen response) was reported in 7 of 15 patients (46.6%). Among the 8 patients with baseline thrombocytopenia, 6 patients (75%) had a platelet response.
What is the clinical relevance of these findings?
Dr. Prithviraj Bose: We saw a nice increase in platelets. That has been a particularly difficult aspect of this disease to improve, and we're seeing some very nice platelet responses which we attribute to the thalidomide. Importantly, we have used a low dose, in fact, Dr. Mesa, you are one of the pioneers of this many years ago, with doses of thalidomide at 50 mg/day in combination with ruxolitinib, and we have, of course, seen responses in other disease parameters as well. But then I think the main contribution of thalidomide appears to be in the platelets.
Dr. Ruben Mesa: Well, I think it's a very important observation coming from the study, having been involved with the early thalidomide studies. There were many patients who had been treated with low doses of thalidomide and prednisone and having efficacy. I think it's a natural combination as well as potentially being an accessible combination for many patients around the world.
Ruxolitinib Plus Pomalidomide in MF Patients with Anemia: Updated Results from the German MPNSG-0212 Trial
The combination of ruxolitinib and pomalidomide is under investigation in an ongoing, multi-center, open-label, single-arm, phase 1b/2 study.3 The study aim is to evaluate the potential synergism of ruxolitinib plus pomalidomide to improve anemia and quality of life (QoL) in patients with MF. The trial was designed to include a target population of 90 patients in a two-stage design: patients in Cohort 1 received ruxolitinib 10 mg BID plus low-dose pomalidomide 0.5 mg QD, and those in Cohort 2 received a step-wise dose increase of pomalidomide from 0.5 mg to 1 mg and 2 mg QD after three and six 28-day cycles, respectively. The main inclusion criterion was MF with anemia (Hb <10 g/dL and/or RBC transfusion dependence [RBC-TD]). The trial excluded patients who were eligible for allogeneic transplantation and those with low platelet counts (<100/nL).
Sixty-seven of the 79 patients included at data cut-off were evaluable (Cohort 1, n=40; Cohort 2, n=27).3 Efficacy results showed that clinical improvement [Hb increase ≥2 g/dL] was achieved in 18% and 20% of patients in Cohorts 1 and 2, respectively. Notably, 42% of all patients were treated with >12 cycles and showed a longer lasting stabilization of their disease with sustained improvement of Hb and QoL.
What do these findings mean to clinical practice?
Dr. Ruben Mesa: It is an interesting study that contrasts nicely with the thalidomide study. If I were considering off-label use of ruxolitinib with an immunomodulatory drug in 2020, I think I'd be more inclined to use thalidomide. Both of our centers participated with the single-agent pomalidomide studies in MF, and although it was active, it was not active enough to become approved on its own. I suspect that with the expense associated with pomalidomide, one of our other novel combination studies with a BET inhibitor or something else will likely end up becoming more attractive than utilizing pomalidomide.
“Add-on” Ruxolitinib Therapy
MANIFEST: CPI-0610 in Ruxolitinib-Refractory or -Intolerant MF
CPI-0610 is a selective and potent small molecule BETi that has been shown clinical activity and a wide therapeutic window in a phase 1 study in lymphoma.4 This agent is being studied in the phase 2 MANIFEST trial as monotherapy or in combination with ruxolitinib, in ruxolitinib-refractory or -resistant MF (Figure 1).
Figure 1. MANIFEST Study Schema4
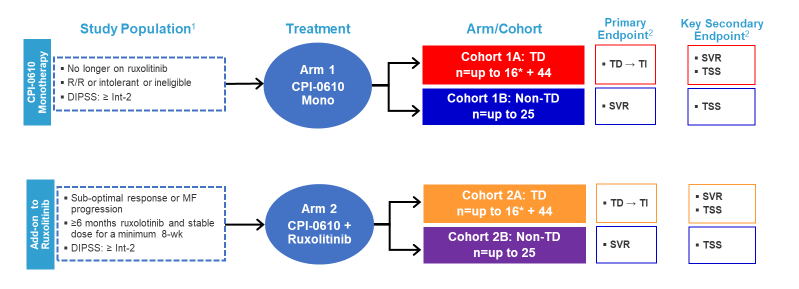
1ClinicalTrials.gov Identifier: NCT02158858 for further details on study design and patient population per last protocol amendment.
2Other endpoints: Anemic response, RBC transfusion rate, safety, PK, proinflammatory cytokine levels, bone marrow morphology and mutant allele burden.
*Will follow Simon 2-stage design.
DIPSS=Dynamic International Prognostic Scoring System; MF= myelofibrosis; R/R=relapsed/refractory; TD=transfusion dependent; TI=transfusion independent; SVR=spleen volume response; TSS=total symptom score; wk=week
Results showed that spleen volume response (SVR) among transfusion-dependent (TD) patients (n=12) was 25% and 0% in those who were non-transfusion dependent (non-TD; n=13).4 More patients who were non-TD and on CPI-0610 monotherapy (n=11) had greater improvements in hemoglobin versus those receiving the combination regimen (n=15; 55% vs 13%, respectively). Finally, improvement in bone marrow fibrosis was also reported; 12 of 32 patients (38%) had at least a grade 1 improvement in bone marrow fibrosis, and 10 of 12 (83%) improvements occurred within the first 6 months of therapy. Data indicate that more patients on the combination regimen had an improvement in bone marrow fibrosis versus those on the monotherapy arm (Table 1).
Table 1. Improvement in Bone Marrow Fibrosis in the MANIFEST Trial4
| Treatment Arm, n/N (%) |
BMF Improvement1 | BMF Improvement and Hemoglobin Increase2 |
BMF Improvement and TD to TI |
| CPI-0610 monotherapy | 2/9 (22.2) | 2/9 (22.2) | 0.2 (0) |
| CPI-0610 add-on to ruxolitinib | 10/23 (43.5) | 4/23 (17.4) | 4/11 (36.4) |
1Bone marrow evaluable population: Baseline and at least one post-baseline bone marrow biopsy at or after 24 weeks available. Data assessed by local labs per the European consensus on grading bone marrow fibrosis and assessment of cellularity.5
21.5 g/dL hemoglobin increase from baseline. Received treatment for ≥12 weeks; mean+/-SEM. The increases in hemoglobin were confirmed within 6 weeks.
BMF=bone marrow fibrosis; TI=transfusion independent; TD=transfusion dependent
What are the potential implications of these findings to practice?
Dr. Ruben Mesa: I think it's a very intriguing set of data without question, I mean, the drug clearly appears to be active. I would be cautious just by the numbers in terms of putting too much weight on whether there is an efficacy difference between transfusion-dependent and non-transfusion-dependent patients. It really may just be that phenomenon. All of that said, there surely could be a biological difference that we don't yet appreciate, as you know there are patients who have a stable anemia with a hemoglobin of 8.5 g/dL and can have that for years, whereas others who are transfusion dependent right away. Do they have a difference in biology? I think only time will tell, but some interesting data without question.
Navitoclax Combined with Ruxolitinib in Primary or Secondary MF
Navitoclax is a novel small molecule that binds with high affinity to BCL-XL, BCL-2, and BCL-W, causing cell death by apoptosis,6 and has shown cytotoxic activity in MPN-derived cell lines.7-10 This agent was investigated in a phase 2, single-arm open-label trial in combination with ruxolitinib in patients with primary or secondary MF (Figure 2).11 The trial was based on the hypothesis that the addition of navitoclax to ruxolitinib would overcome resistance to JAK2 inhibitors.
Figure 2. Navitoclax as Add-on Therapy to Ruxolitinib: Phase 2 Study Design11
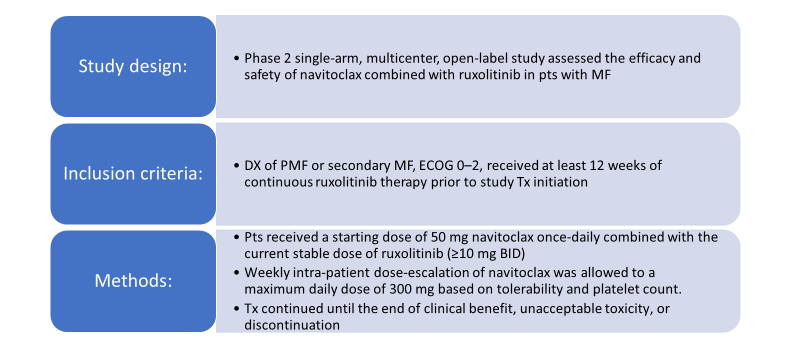
BID=twice daily; DX=diagnosis; ECOG=Eastern Cooperative Oncology Group; MF=myelofibrosis; PMF=primary myelofibrosis; pts=patients; Tx=therapy
Findings showed that 23 of the 34 patients enrolled on the study achieved the 300-mg dose of navitoclax, and 22 of 25 patients who enrolled on ruxolitinib at doses of >10 mg BID had ruxolitinib dose-reduced to 10 mg BID.11 About a third of the 24 evaluable patients had an SVR ≥35%, and 20% of patients had an improvement in total symptom score (TSS). Reductions in driver mutation allelic burden of >5% also were reported, as well as improvements in bone marrow fibrosis. All patients experienced an adverse event of any grade, and 8 patients had a serious adverse event, which included anemia, pancytopenia, splenic infarction, upper abdominal pain, vomiting, chest pain, pneumonia, and abnormal liver function. One patient experienced grade 5 pneumonia, but this was unrelated to navitoclax therapy.
Dr. Ruben Mesa: I liked seeing the incremental benefit that navitoclax provided in patients who were already on ruxolitinib and that it really kicked up the level of response to a greater level. It is encouraging to see and how these data evolve.
Luspatercept in MF-associated Anemia: Open-label Phase 2 Study
Luspatercept is a first-in-class erythroid maturation agent that binds several TGF-β superfamily ligands to diminish Smad2/3 signaling and enhances late-stage erythropoiesis.12 It is approved in the US for use in adult patients with β-thalassemia who require regular red blood cell (RBC) transfusions.13 On April 3, 2020, the agent was approved for use in the treatment of anemia failing an erythropoiesis-stimulating agent and requiring two or more RBC units over 8 weeks in adult patients with very low- to intermediate-risk myelodysplastic syndromes (MDS) with ring sideroblasts or with MDS/MPN with ring sideroblasts and thrombocytosis.14
Luspatercept is under investigation in an open-label phase 2 trial in patients with MF-associated anemia who were either transfusion independent or transfusion dependent (Figure 3).15 Patients who were non-transfusion dependent could not have had red blood cell transfusions within 12 weeks before enrollment and hemoglobin levels of ≤9.5 g/dL, recorded on three separate occasions. Patients who were transfusion dependent could have received 2-4 red blood cell units per 28 days within 12 weeks before enrollment and no transfusion-free intervals of more than 6 weeks.
Figure 3. Study Schema of the Phase 2 Trial of Luspatercept in Myelofibrosis-associated Anemia.15
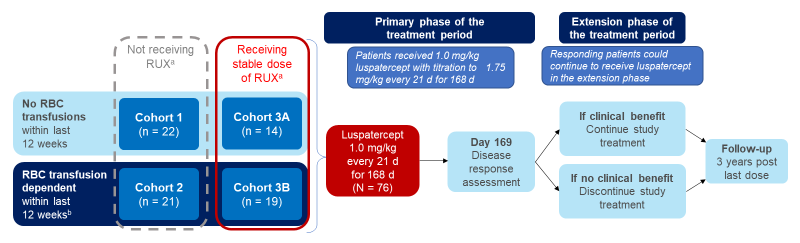
aA stable dose of ruxolitinib for at least 16 weeks at enrollment.
b2-4 RBC U/28 days transfused within the last 12 weeks.
*D=day; RBC=red blood cell; RUX=ruxolitinib
The median duration of luspatercept treatment was 24 weeks (range, 2-86 weeks). Dose escalation to 1.33 mg/kg and 1.75 mg/kg was observed in 65 (86%) and 50 (66%) patients, respectively. Results showed that the combination regimen appeared to be more efficacious vs luspatercept monotherapy (Table 2).15
Table 2. Efficacy Findings from a Phase 2 Trial of Luspatercept in Myelofibrosis-associated Anemia15
| No RBC Transfusionsa | RBC Transfusion Dependenta | |||
| Response Characteristic | Not Receiving RUXb | Receiving RUXb | Not Receiving RUXb | Receiving RUXb |
| Cohort 1
(n = 22) |
Cohort 3A (n = 14) |
Cohort 2 (n = 21) |
Cohort 3B (n = 19) |
|
| Hb increase ≥1.5 g/dL from baseline for ≥12 consecutive weeksc,d | ||||
| Hb increase ≥1.5 g/dL at every assessment | 3 (14) | 3 (21) | – | – |
| Mean Hb increase ≥1.5 g/dL | 4 (18) | 9 (64) | – | – |
| RBC transfusion-free ≥12 consecutive weeks, n (%)e | – | – | 2 (10) | 6 (32) |
| Duration of response, median (range), weeksf | – | – | 32 (16–49) | 39 (12–77) |
| ≥50% reduction in RBC transfusion burden from baseline, n (%)g | – | – | 8 (38) | 10 (53) |
a In the 12 weeks prior to treatment.
b A stable daily dose of ruxolitinib for ≤16 weeks at enrollment.
c Per central laboratory assessment.
d Three subjects have an ongoing response at the time of data cutoff.
e Six subjects have an ongoing response at the time of data cutoff.
f Duration of response was defined as the duration of the longest single response period.
g Minimum four RBC U decrease
What is the clinical relevance of these findings to practice?
Dr. Ruben Mesa: What about the combination? Ruxolitinib is typically thought of as having drug-emergent anemia. So, the fact that the combination was a bit more beneficial is interesting in itself. Likewise, we saw individuals who were transfusion dependent have perhaps a bit more benefit than those who were not. Is there a biological clue there? Is it just the impact of small numbers? It's difficult to know. But I think the takeaway is that there certainly is activity of luspatercept potentially in MF with ruxolitinib, and certainly, there are planned prospective studies to try to further elucidate that and see whether the indication should be expanded for luspatercept and could also be considered for patients with MF.
Download References